Benzene sulfonation is an electrophilic aromatic substitution (EAS) reaction where a sulfonic acid group (-SO₃H) is introduced into the benzene ring. The reaction is typically carried out using sulfur trioxide (SO₃) and concentrated sulfuric acid (H₂SO₄).
Sometimes, this reaction is written as "fuming H2SO4" or "Oleum". The terms "fuming sulfuric acid" and "oleum" are used to describe sulfuric acid that contains a significant amount of dissolved sulfur trioxide (SO₃). They are typically used by old-school professors and here’s why these terms are used:
Fuming Sulfuric Acid
Fuming sulfuric acid refers to sulfuric acid that is saturated with sulfur trioxide (SO₃). This solution is called "fuming" because when exposed to air, it releases white fumes of sulfur trioxide, which are highly reactive and can form sulfuric acid upon contact with moisture in the air. The presence of these fumes gives the solution its characteristic "fuming" appearance.
Oleum
Oleum is another name for fuming sulfuric acid. It specifically refers to a solution of sulfur trioxide (SO₃) in sulfuric acid (H₂SO₄). Oleum is usually represented as H₂SO₄·xSO₃, where "x" indicates the amount of sulfur trioxide dissolved in the sulfuric acid. For example, if the solution contains an equimolar amount of sulfur trioxide and sulfuric acid, it might be denoted as H₂SO₄·SO₃.
Here are examples of the sulfonation when the benzene has an electron withdrawing group (EWG) and electron donating group (EDG):
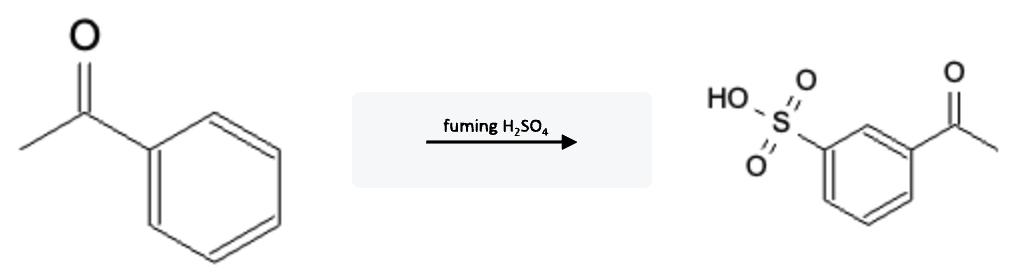
Nitration Electron Withdrawing Group (EWG)
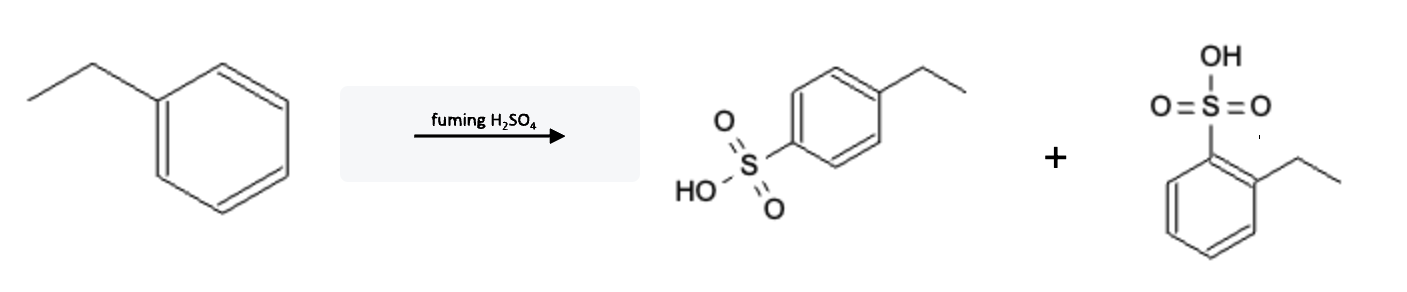
Nitration Electron Donating Group (EDG)
Mechanism
The reaction mechanism is depicted below:
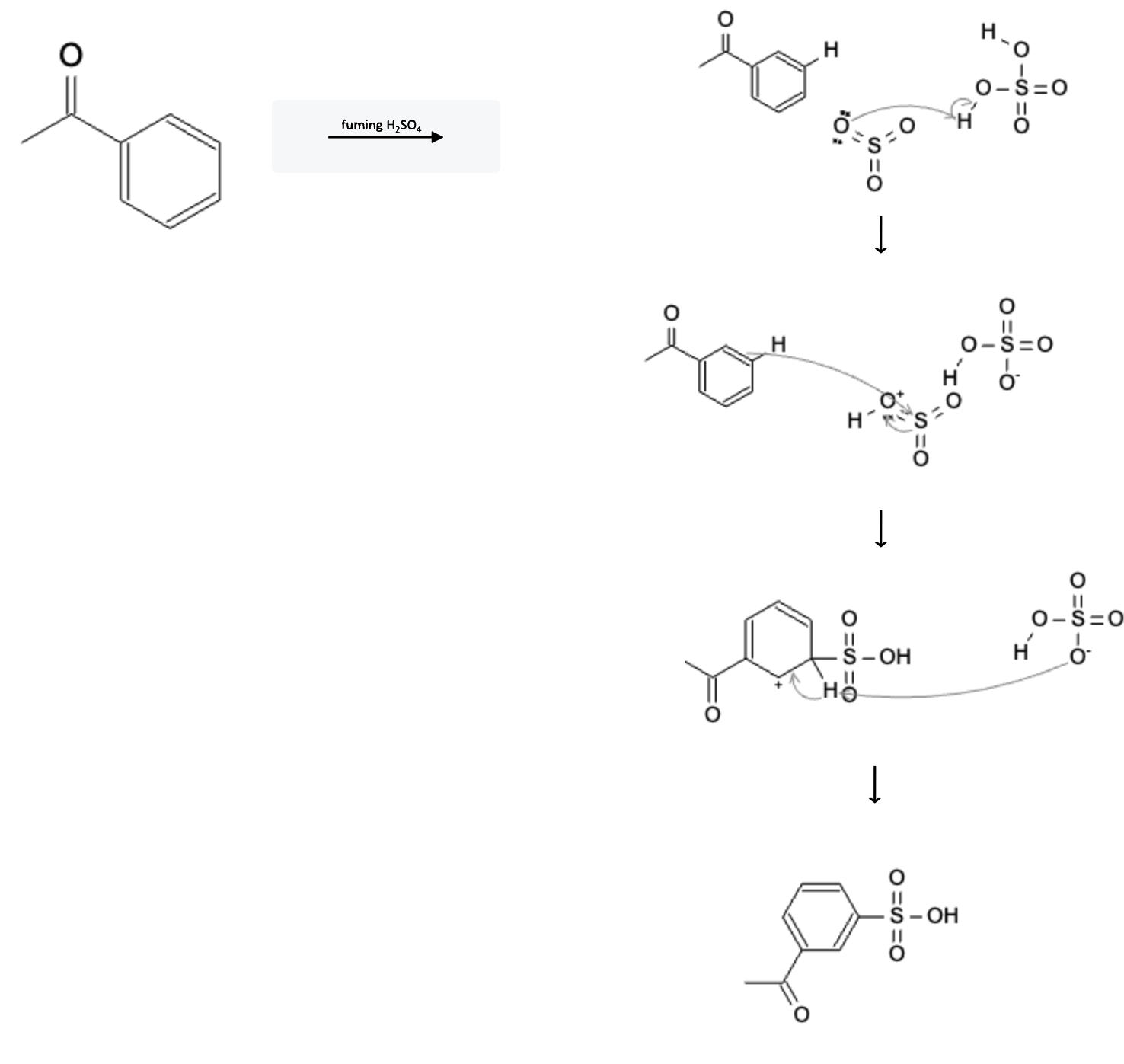
In the first step, the SO3 molecule reacts with the H2SO4, protonating the SO3 molecule resulting in a negatively charged HSO4- molecule.
In the second step, the pi electrons from a benzene double bond attack the sulfur atom of the HSO3 molecule. This sends the pi electrons from the S=O double bond over to the O atom.
In the third step, the negatively charged HSO4- molecule from the first step pulls the hydrogen atom off the benzene ring, sending the hydrogen bond electrons back to the benzene ring, forming the final product.
Practice this reaction using our Reaction Solver!


